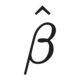Biostatistics stands as an indispensable element in the landscape of clinical research and trials, transcending mere post-analysis applications. Its roots trace back to the 17th century, evolving into a pivotal force in contemporary clinical research. In today’s context, biostatistics takes center stage, guiding the articulation of scientific manuscripts through intricate statistical analyses of medical data. Beyond the realm of post-analysis, the active involvement of biostatisticians throughout the clinical trial process, from inception to outcome reporting, underscores their critical role in ensuring rigor and precision.
Role of Biostatistics in Clinical Research and Trials
Biostatistics plays a pivotal role at every stage of clinical research, influencing the design, execution, analysis, and interpretation of clinical trials. Below is an overview of how biostatistics is integral to each phase of clinical research:
- Trial Design: Biostatisticians actively contribute to shaping the overall structure of the clinical trial. This involves selecting an appropriate study design, such as a randomized controlled trial, determining the sample size, and defining primary and secondary endpoints. They play a crucial role in randomization processes to ensure impartial allocation of participants to treatment groups. Key considerations during trial design include statistical power and minimizing bias.
- Protocol Development: In the development of the trial protocol, biostatisticians articulate the hypothesis to be tested and make decisions regarding the selection and endpoints of the trial. They actively participate in determining the study design, including its type, schematic representation, techniques, and stages. Protocols are meticulously crafted to minimize or eliminate bias, with biostatisticians ensuring the appropriate incorporation of statistical methodologies.
- Data Management: Biostatistics is instrumental in creating the data management plan, guaranteeing the production of high-quality data with minimal errors throughout the clinical trial. They contribute to decisions on data collection methods, define data cleaning processes, and establish strategies for handling missing data.
- Data Analysis: Biostatisticians leverage statistical methodologies to analyze intricate datasets from clinical trials, uncovering patterns and drawing meaningful conclusions. Various statistical tests, such as t-tests or chi-square tests, are employed to compare outcomes between treatment and control groups. For studies evaluating time-to-event data, techniques like survival analysis may be applied.
- Reporting: Throughout the reporting phase, biostatisticians are actively engaged in interpreting and presenting the results of the clinical trial. They ensure accurate communication of statistical significance, effect sizes, and clinical relevance. Biostatisticians contribute significantly to preparing the statistical sections of study reports, publications, and regulatory submissions. Their involvement ensures a comprehensive and precise representation of the trial outcomes.
Common Biostatistics Methods in Clinical Research
- Sample Size Determination: Calculating the appropriate number of participants based on statistical power is crucial for detecting treatment effects accurately. Biostatisticians use various formulas and considerations to determine an optimal sample size that ensures the trial’s validity and reliability.
- Descriptive Statistics: Summarizing and presenting safety-related and efficacy-related data is a fundamental aspect of clinical research. Biostatisticians employ descriptive statistics, including measures such as means, standard deviations, and frequencies, to provide a concise overview of the observed data.
- Inferential Statistics: Inferential statistics play a key role in drawing conclusions about safety parameters and treatment effects. Techniques such as confidence intervals and hypothesis testing are employed to make inferences and generalize findings from the observed sample to the broader population under study.
- Modeling Approaches: Statistical modeling is applied to analyze pharmacokinetic and pharmacodynamic data, enabling the prediction of dosage-response relationships. Biostatisticians use various modeling techniques to understand and quantify the relationships between drug doses, biological responses, and other relevant variables.
- Bayesian Methods: Bayesian statistical approaches provide flexibility by incorporating prior information into clinical trials. Biostatisticians may utilize Bayesian methods to update probabilities and make informed decisions based on both existing data and prior knowledge, offering a comprehensive perspective in the analysis of clinical trial outcomes.
Types of Clinical Trials
Interventional Trials:
- Objective: Evaluate the effects of a medical product, intervention, or treatment.
- Phases: I, II, III, IV.
- Statistical Techniques:
- Phase I: Descriptive statistics for safety and dose escalation.
- Phase II: Preliminary efficacy analysis, determining the optimal dose.
- Phase III: Confirmatory analysis for efficacy and safety.
- Phase IV: Post-marketing surveillance and long-term safety.
Observational Trials:
- Objective: Observe and collect data on participants without intervention.
- Types: Cohort studies, case-control studies, cross-sectional studies.
- Statistical Techniques: Descriptive statistics, regression analysis, propensity score matching.
Expanded Access (Compassionate Use) Trials:
- Objective: Provide access to investigational treatments outside of clinical trials.
- Statistical Techniques: Limited, mainly descriptive statistics.
Each type of clinical trial serves distinct purposes, utilizing specific methodologies tailored to its objectives. Interventions trials assess the impact of treatments, observational trials observe participants without interventions, and expanded access trials provide access to investigational treatments outside the standard trial framework. The statistical techniques applied vary accordingly, ensuring meaningful analysis and interpretation of outcomes.
Phases of Clinical Trials
Clinical trials are categorized into different types and phases, each with its specific objectives, designs, and statistical considerations. Here’s an overview of the types and phases of clinical trials along with the associated statistical techniques:
Phase I: Phase I clinical trials typically involve healthy volunteers, i.e., individuals without known health conditions. Generally, a cohort of 20 to 80 healthy volunteers participates in this initial phase. The primary purpose of Phase I is to assess how the drug functions within the human body and to identify any side effects linked to escalating dosages.
- Objective: Assess safety, dosage range, and identify side effects.
- Statistical Techniques:
- Descriptive statistics for safety outcomes.
- Determination of Maximum Tolerated Dose (MTD).
Phase II: Phase II trials typically involve a larger participant pool compared to Phase I. This stage focuses on an in-depth examination of safety measures and evaluates the efficacy of the drug. Occasionally, Phase II trials may compare a novel treatment with an existing one or a placebo. These trials often target specific groups with a particular disorder, diverging from the broader healthy volunteer approach in Phase I. Successful outcomes in Phase II may pave the way for further testing in Phase III clinical trials.
- Objective: Assess efficacy and side effects in a larger group.
- Statistical Techniques:
- Preliminary analysis of efficacy outcomes.
- Estimation of response rates.
Phase III: Phase III serves the purpose of comparing a new drug with the standard-of-care drug, meticulously evaluating the side effects and determining the drug’s comparative effectiveness. These trials commonly incorporate randomization, where participants are assigned to treatment groups without bias. Typically, Phase III studies engage 300 to 3,000 participants from a specific patient population for which the medicine is intended. Often deemed pivotal trials, Phase III clinical trials are crucial in obtaining FDA approval for public drug use. Approval from Phase III trials is fundamental, granting doctors the authorization to prescribe the medication for their patients.
- Objective: Confirm efficacy and monitor adverse reactions.
- Statistical Techniques:
- Randomized controlled trials (RCTs) with larger sample sizes.
- Hypothesis testing for primary and secondary endpoints.
- Meta-analysis for combining results from multiple trials.
Phase IV: Phase IV serves as a critical stage for testing new drugs approved by the FDA. During this phase, the drug is administered to several hundred or even thousands of patients. This extensive testing aids in identifying both short-lived and enduring side effects, ensuring a thorough assessment of safety in the broader patient population. In some instances, rare side effects may only become apparent within large groups. Additionally, Phase IV allows doctors to gain deeper insights into the drug’s effectiveness and its compatibility when used alongside other treatments.
- Objective: Monitor long-term safety and effectiveness after approval.
- Statistical Techniques:
- Pharmacovigilance using real-world data.
- Post-marketing surveillance studies.
Specific Statistical Considerations in Clinical Research and Trials
- Randomization: Minimizes bias and ensures comparable treatment groups.
- Blinding: Single-blind or double-blind design to prevent bias in outcome assessment.
- Placebo-Controlled Trials: Used to assess the true effect of the intervention.
- Crossover Trials: Participants receive multiple treatments in a randomized order.
- Adaptive Trial Designs: Flexibility to modify the trial based on interim analyses.
- Survival Analysis: Time-to-event analysis for studies with endpoints like progression-free survival.
- Interim Analyses and Data Monitoring Committees: Ensure ethical conduct and integrity of the trial.
- Subgroup Analyses: Assess treatment effects in specific subpopulations.
Application of Biostatistics in Real-World Clinical Problems
Grasping the intricacies of statistical design and analysis is imperative for deriving credible conclusions from clinical trials, shaping evidence-based medical practices and healthcare decisions. The following examples and case studies illuminate the practical application of biostatistics in addressing real-world clinical challenges, shedding light on both successful outcomes and encountered challenges:
Randomized Controlled Trials (RCTs) in Drug Development:
- Objective: To determine the efficacy and safety of a new drug.
- Successes:
- RCTs have led to the approval of numerous life-saving drugs, such as statins for cardiovascular diseases.
- Rigorous randomization and blinding reduce biases and provide robust evidence.
- Challenges:
- Ethical considerations in placebo-controlled trials, especially in life-threatening conditions.
- Recruitment challenges and participant dropout affecting statistical power.
Adaptive Trial Design in Cancer Research:
- Objective: Optimize treatment strategies based on interim analyses.
- Successes:
- Adaptive designs allow for flexibility, potentially leading to faster drug development.
- Improved patient outcomes with adaptive adjustments to treatment arms.
- Challenges:
- Complex statistical methodologies required for adaptive design implementation.
- Increased risk of Type I errors if not properly controlled.
Survival Analysis in Oncology Trials:
- Objective: Assess time-to-event outcomes like overall survival.
- Successes:
- Kaplan-Meier curves provide a clear visualization of survival probabilities.
- Cox proportional hazards model helps identify factors influencing survival.
- Challenges:
- Censoring of data complicates analyses and interpretation.
- Assumptions of proportional hazards may not always hold.
Meta-analysis of Clinical Trials:
- Objective: Combine results from multiple trials to obtain a more robust estimate of treatment effects.
- Successes:
- Meta-analyses can provide a more comprehensive view of treatment efficacy.
- Quantifies heterogeneity between studies.
- Challenges:
- Variability in study designs, populations, and endpoints.
- Publication bias and selective reporting can influence results.
Longitudinal Data Analysis in Diabetes Research:
- Objective: Assess changes in outcomes over time in diabetes interventions.
- Successes:
- Mixed-effects models handle repeated measures and account for individual variability.
- Identification of trends and patterns in long-term interventions.
- Challenges:
- Missing data and irregular follow-ups can impact longitudinal analyses.
- Complex models may be challenging for non-statisticians to interpret.
These examples demonstrate the versatility and impact of biostatistics in diverse clinical settings. While biostatistics plays a crucial role in generating evidence-based insights, addressing challenges such as ethical considerations, trial complexities, and data variability is essential for ensuring the validity and reliability of study findings.
Books and Articles for Further Information
Below are some recommended courses, books, and articles that individuals can explore to improve their knowledge:
Courses:
- University of Michigan – Statistics with Python Specialization
- Provider: Coursera
- Description: Master statistical analysis with Python: explore data collection, design, management, and create powerful visualizations.
- Osaka University – Introduction to Applied Biostatistics: Statistics for Medical Research
- Provider: edX
- Description: Analyze Medical Data with R Commander: Hands-On Practice.
- UTMB – Biostatistics for Big Data Applications
- Provider: edX
- Description: Master Data Analysis for Biomedical Research with Hands-On R Practice.
- Stanford University – Machine Learning Specialization
- Provider: Coursera
- Description: Learn the fundamentals of machine learning and build real-world AI applications with this beginner-friendly program from DeepLearning.AI and Stanford Online…
Books:
- “Principles of Biostatistics” by Marcello Pagano and Kimberlee Gauvreau. A comprehensive textbook covering the fundamental principles of biostatistics with practical examples.
- “Statistical Methods in Medical Research” by Peter Armitage and Geoffrey Berry. It provides a detailed overview of statistical methods commonly used in medical and clinical research.
- “Biostatistics: A foundation for Analysis in the Health Sciences” by Wayne W. Daniel and Chad L. Cross. A textbook covering basic biostatistical concepts and their applications in health sciences.
Articles and Journals:
- “Statistics in Medicine” Journal – A peer-reviewed journal publishing articles on the statistical methods used in medical and clinical research.
- “Biometrics” Journal – Focuses on the development and application of statistical and mathematical methods in biology and medicine.
- “Journal of Clinical Epidemiology” – Covers topics related to clinical research, epidemiology, and biostatistics.
- “The American Statistician” – A journal covering a wide range of statistical methodologies, including those applicable to medical and clinical research.
Other Online Resources:
- National Institutes of Health (NIH) – Biostatistics Resources: This platform provides diverse resources, courses, and tools curated by the NIH to augment comprehension of biostatistics.
- Statistics.com – Biostatistics Courses: Statistics.com offers a variety of online courses specifically designed for biostatistics, focusing on its applications in health research.
These resources serve as a well-rounded foundation for individuals seeking to enhance their knowledge and skills in biostatistics for clinical research. Whether opting for formal courses, delving into textbooks, or exploring academic journals, a combination of these resources contributes to a comprehensive understanding of biostatistics within the realm of clinical research.
Conclusion
In the dynamic landscape of clinical research, biostatistics emerges not just as a tool for analysis but as a driving force shaping the trajectory of trials. From designing trials and developing protocols to managing data and reporting outcomes, biostatisticians navigate each phase, infusing statistical rigor. The diverse array of statistical methods, coupled with their application in real-world challenges, underscores the robustness and adaptability of biostatistics. As we delve into its nuances, the knowledge gleaned from biostatistics becomes not just a necessity but a compass, guiding evidence-based medical practices and healthcare decisions.